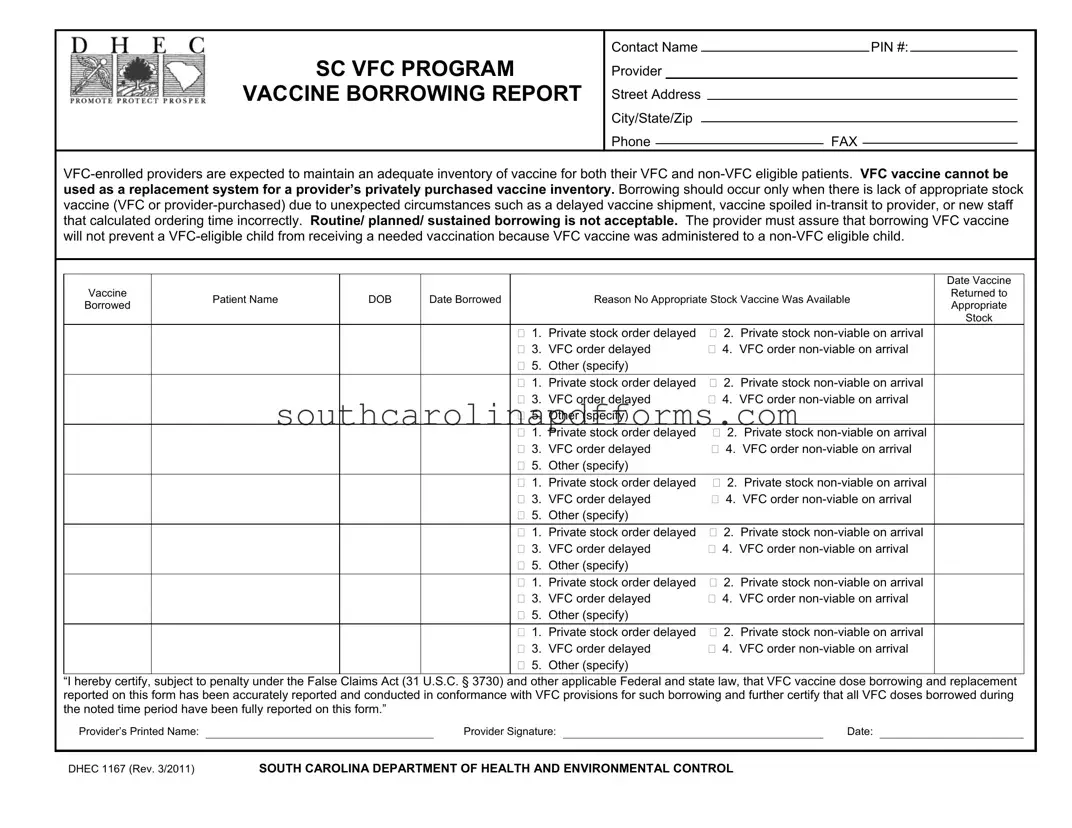
The DHEC 1167 form, also known as the SC VFC Vaccine Borrowing Report, plays a crucial role in ensuring that healthcare providers maintain appropriate vaccine inventories for both VFC and non-VFC eligible patients. This form is essential for documenting instances when a provider must borrow vaccines due to unexpected circumstances, such as delayed shipments or spoiled stock. It emphasizes that borrowing should not become a routine practice; rather, it should only occur in genuine emergencies. The form requires detailed information, including the provider's identification number, contact details, and specific data about each borrowed vaccine, such as the patient's name and the reason for borrowing. Providers must also certify that all borrowed doses are accurately reported and comply with VFC guidelines. Proper record-keeping is mandated, with the expectation that completed forms are retained for three years, ensuring accountability and transparency in vaccine management.