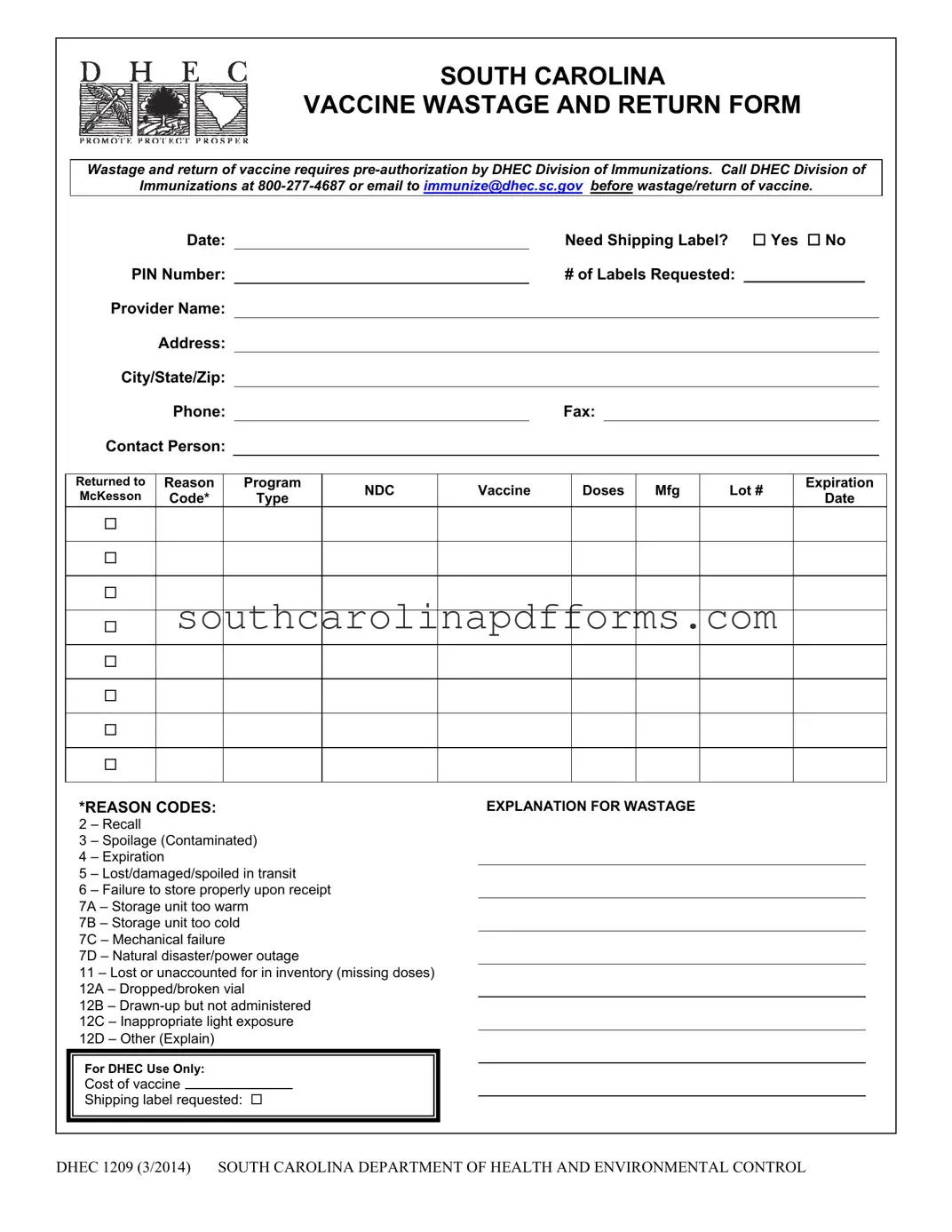
The DHEC 1209 form, officially known as the South Carolina Vaccine Wastage and Return Form, plays a crucial role in managing vaccine wastage and returns within the state. This form ensures that any wastage or return of vaccines is properly documented and authorized by the South Carolina Department of Health and Environmental Control (DHEC) Division of Immunizations. Before any vaccine can be wasted or returned, providers must seek pre-authorization from DHEC, either by calling their designated hotline or sending an email. The form requires essential information from the provider, including contact details and specifics about the vaccines involved, such as the reason for wastage, manufacturer, lot number, and expiration date. Additionally, providers must indicate whether they need shipping labels for returning vaccines to McKesson, the central distributor for the Centers for Disease Control and Prevention. Detailed instructions guide providers through the completion process, emphasizing the importance of accurate reporting and retention of the form for compliance with federal regulations. Ultimately, the DHEC 1209 form is a vital tool for ensuring the safe and responsible management of vaccines, thereby supporting public health initiatives across South Carolina.